CO2EXIDE – Technology
The biogenic off gas CO2 from a biogas plant acts as a carbon source for the project. The CO2 will be captured, purified and stored as it is routine throughout biomethane production (AXIOM). The captured CO2 will then be fed into an electrochemical reactor, which is based on PEM-Cell design (Siemens, SG). The cathodic half-cell will be fed with CO2 and catalyst containing gas diffusion layers will reduce CO2 to produce ethylene (AGH, ISSP, SOTON, BME). The anodic feed consists of an aqueous electrolyte as feedstock for the direct oxidation of water to hydrogen peroxide (SOTON, Fraunhofer, BME). Ethylene will be separated from the other cathodic products (H2, CO, CH4) for safety reasons, to avoid a mixture of H2 and O2 in the subsequent chemical conversion step. (AXIOM, Siemens). The produced ethylene and hydrogen peroxide will finally be fed into a chemical cascade reactor and converted into ethylene oxide and different polyethylene glycols (Fraunhofer).
To enable a truly sustainable and circular economy, the utilization of carbon dioxide (CO2) as raw material (Carbon Capture and Utilization, CCU) is an important technical approach. In this context, the central objective of the CO2EXIDE project was the development of a sustainable alternative process for the production of the bulk chemical ethylene oxide (EO). The proposed technology consists of the combination of cathodic and anodic electrochemical processes (“simultaneous electrochemical factories”) driven by renewable energy and utilizing water and CO2 as only raw materials. More specifically, the CO2EXIDE process chain (Figure 1) comprises:
i) the supply of raw materials, in particular CO2 from biogenic sources (a biogas plant);
ii) coupled electrocatalysis: the simultaneous electrocatalytic conversion of CO2 into ethylene (cathode) and of water into hydrogen peroxide, H2O2 (anode);
iii) the separation and purification of the generated ethylene; and
iv) the chemical reaction of the purified ethylene with the anodically generated H2O2 to the platform chemical ethylene oxide.
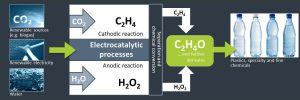
Figure 1: The CO2EXIDE process chain.
In the final phase of the CO2EXIDE project, a demonstrator unit was assembled and tested in the laboratory of the Academic Centre of Materials and Nanotechnology (AGH University of Science and Technology, Kraków, Poland). Figure 2 shows the main hardware components of the final demonstrator: (i) biogenic CO2 sourced from a biogas upgrading plant, (ii) the Electrocatalytic Reactor Unit (ERU), (iii) the Ethylene Enrichment Unit (EEU) and (iv) the Ethylene epOxidation Unit (EOU).
The hardware assembling at AGH was thoroughly planned with simulation and pre-testing. This included (i) drawings of Piping and Instrumentation Diagrams (P&IDs), (ii) execution of Hazard and Operability (HAZOP) Analysis and (iii) testing of all demonstrator units individually prior to installation in the demonstrator setting at AGH laboratory in Kraków (Poland). Therefore, basic testing and process development of Electrocatalytic Rector Unit (ERU) has been carried out at Siemens Energy (Munich, Germany), Ethylene Enrichment Unit (EEU) at AXIOM (Ebreichsdorf, Austria) and Ethylene epOxidation Unit (EOU) at Fraunhofer (Straubing, Germany). Due to COVID-19-related travel restrictions and safety reasons, the EOU could not be transported to and commissioned in Kraków and was operated by Fraunhofer in Straubing (Germany) instead.

Figure 2: The hardware components to demonstrate the CO2EXIDE process chain.
The ERU was based on a 3-chamber-cell-design well suited for the combination of the cathodic conversion of CO2 to ethylene (C2H4) and the anodic conversion of water (H2O) to hydrogen peroxide (H2O2). The cathode was built as Gas Diffusion Electrode (GDE) with sputter-deposited copper, whereas the anode was made of boron-doped diamond (BDD). Within the CO2EXIDE project, a small-size PEM cell with an active area of 25 cm2 and a large-size PEM cell with an active area of 300 cm2 were developed.
The general purpose of the Ethylene Enrichment Unit (EEU) in the CO2EXIDE process is to separate the undesired by-products in the product gas mixture of the ERU and thus to generate an ethylene-enriched gas stream, suitable for further conversion into ethylene oxide in the EOU. For the ethylene enrichment, the process of gas permeation on polymeric membrane is employed. EEU, which is implemented in the WP6 demonstrator, comprises a compressor, a drying and pretreatment section and a one-stage membrane section equipped with highly selective glassy polyimide fibres.
The last step in the process chain is the EOU, which comprises the ethylene epoxidation to Ethylene Oxide (EO). In this process step, the ethylene-enriched gas stream coming from the cathodic compartment of the ERU via EEU and the anodically generated H2O2 dissolved in the anolyte are reacted in the presence of an MWW-type titanosilicate catalysts.
The reaction is carried out in a 2 liter autoclave reactor at mild temperatures and under moderately elevated pressure.
The CO2EXIDE process chain from CO2 (sourced from a biogas plant) to ethylene oxide was successfully demonstrated. This was a joint effort of 10 academic and industrial partners sharing the same vision of a sustainable and CO2-neutral circular economy.






